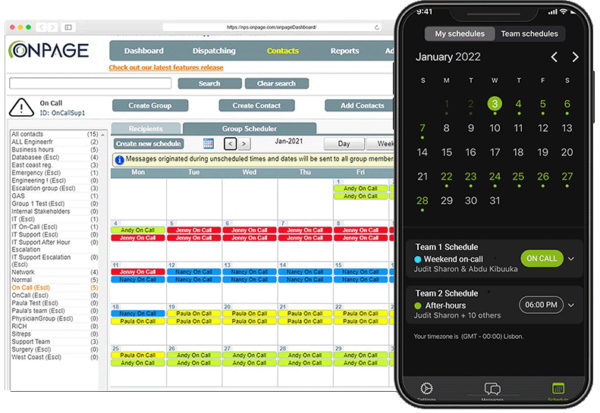
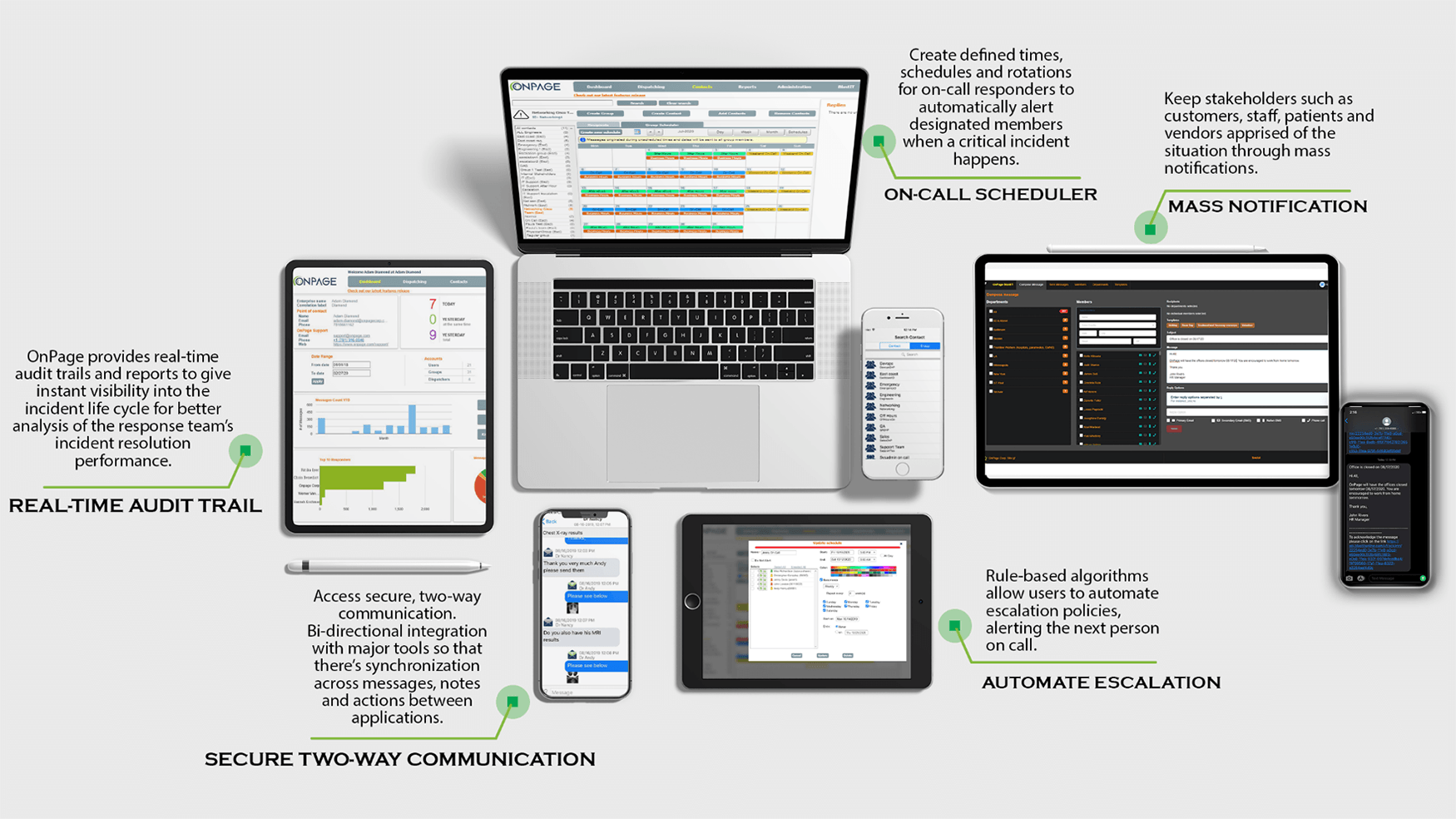
Fail-Safe On-Call Scheduler
Create defined times, schedules and rotations for your on-call response team.
Automatically alert designated members when a critical incident occurs.
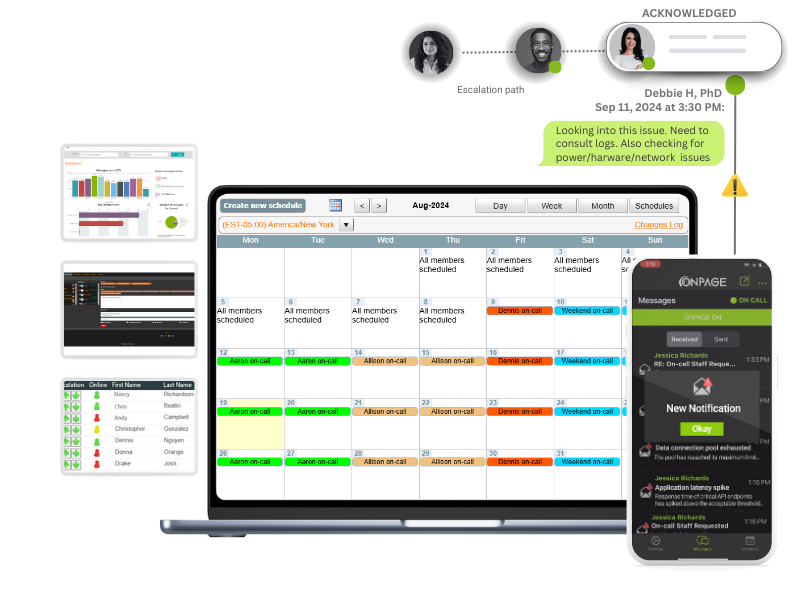
Streamline secure communication and collaboration workflows and drive fast incident remediation for your on-call teams.










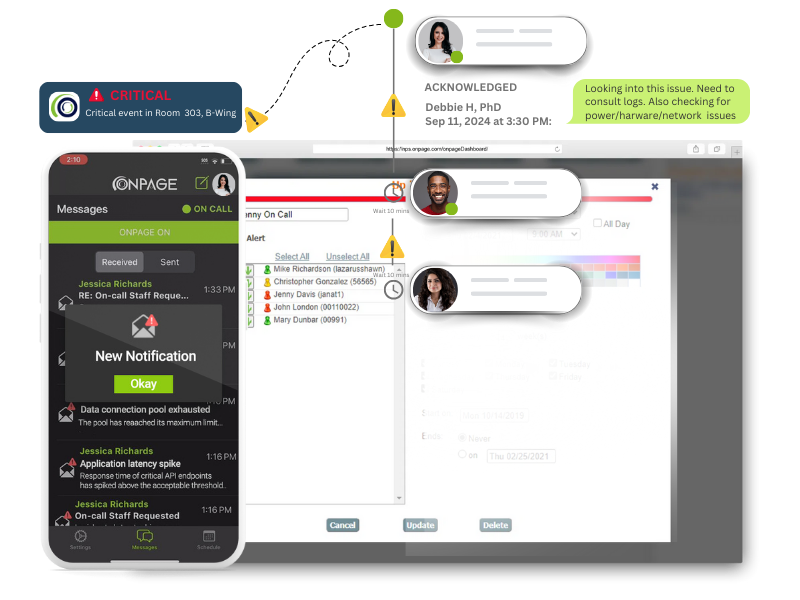
OnPage’s intelligent system centralizes incident response information and elevates critical alerts so your team never misses time-sensitive notifications.
See what OnPage users say on trusted review platforms.
Create defined times, schedules and rotations for your on-call response team.
Automatically alert designated members when a critical incident occurs.

Automate escalation policies to alert the next person on call with OnPage’s rule-based algorithms.
Control the users that will receive the alert and determine the amount of time to wait before escalating critical notifications to the designated user.
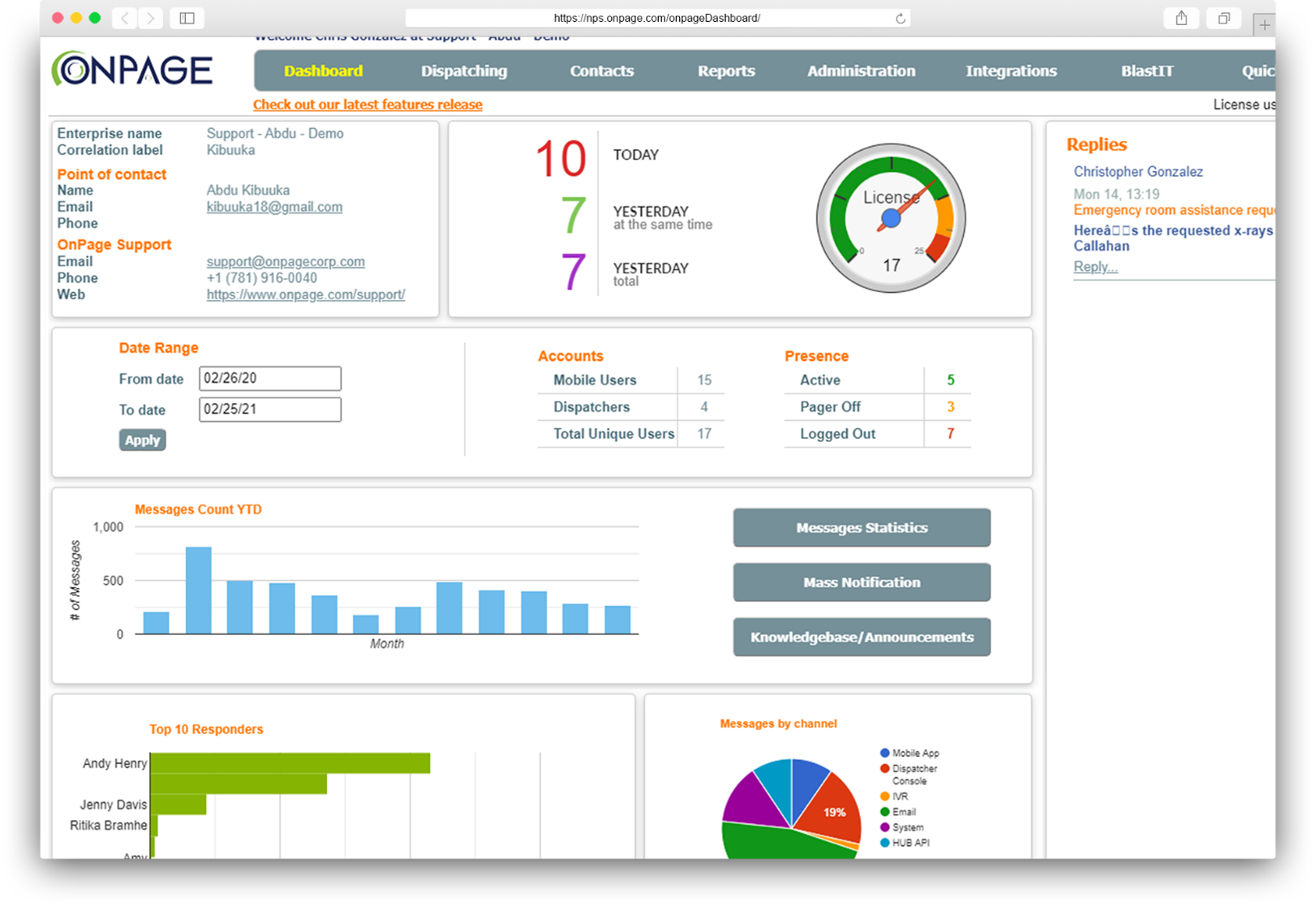
Access real-time audit trails and reports for instant visibility into the incident life cycle.
Strengthen your analysis of the response team’s incident resolution performance.

Bypass the silent switch on all mobile devices. Alerts rise above the clutter® and bring notifications to the forefront, continuing for up to eight hours until acknowledged.
Mobilize your response team with OnPage alerts which are loud and easily distinguishable from other smartphone notifications.
Access secure two-way communications. Messages are SSL encrypted and can only be viewed by message participants. Secure for healthcare and reliable for IT response teams.

OnPage is a G2 Leader for incident alert management, consistently receiving recognition for high performance and user satisfaction. Read more reviews!